What is quality management
Quality management ensures quality is designed into every product and process across the enterprise, including external partners. Quality management provides automated failure mode and effects analysis (FMEA), enables closed-loop corrective and preventive actions (CAPAs) and accurate root cause analysis (RCA) to accelerate identification, containment, and analysis of issues and tracking of affected items. Quality Management is required to achieve compliance with regulatory requirements and quality standards in most industries. However, the complexities of dispersed teams, disconnected systems, and a changing regulatory environment create roadblocks to delivering high-quality products. PTC integrates quality management into the PLM process. This enables better management of quality early in the lifecycle which reduces late stage changes and reduces the costs of poor quality.
Key product features
Unify your engineering, quality, and regulatory teams by introducing best practice change, document, and design control processes.
Change and configuration management: Communicate changes, requirements, test procedures, and manufacturing controls to downstream teams
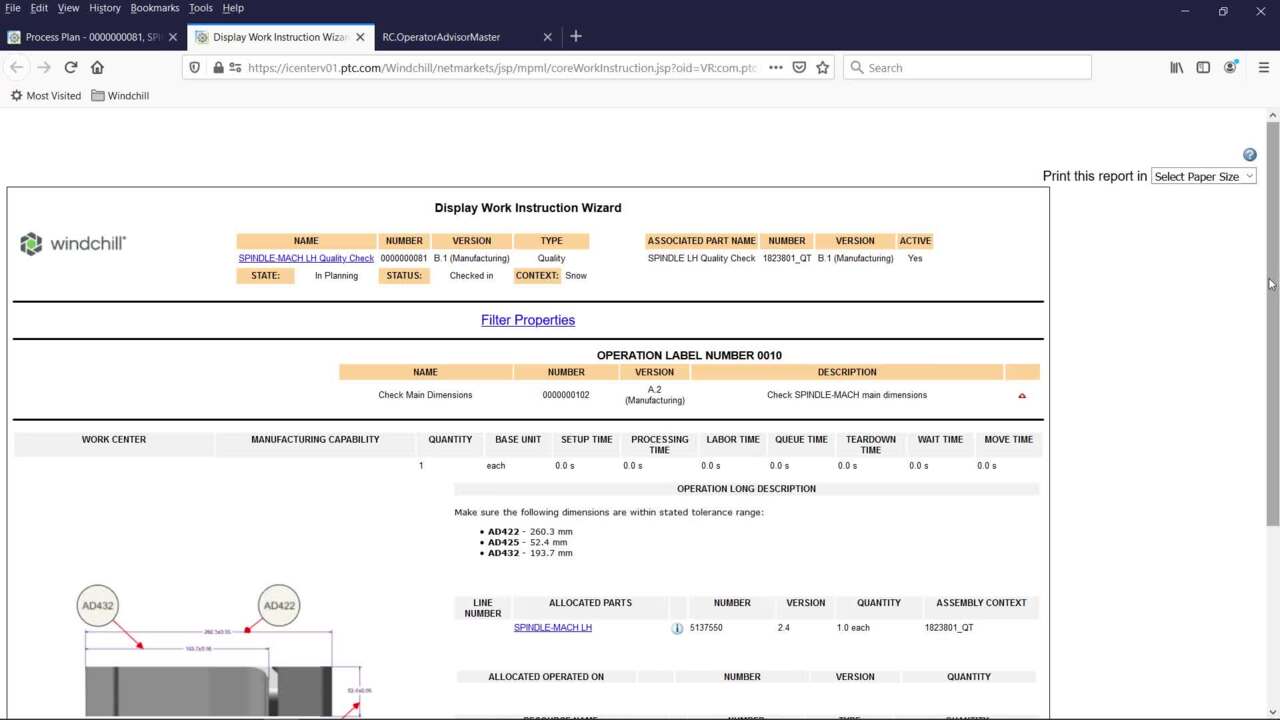
Document control: With integral training tracking
Closed-loop feedback: Collect product failure and performance data from testing, manufacturing, field/IoT for root cause analysis and feedback to design
Audit: Perform internal and supplier audits to ensure that key corporate processes, requirements, and directives are being followed
MBD and MBE: Visibility into model based detailed design data to identify CTQ for development of validation and manufacturing control plans
CAPAs, SCARS, and change requests: Initiate, evaluate, assign, monitor, review, and approve CAPAs arising from internal or external non-conformances
Customer experience management: Capture all customer feedback, including customer complaints
Digital product traceability: Standard OSLC, supports certification and qualification ISO26262, DO-178, etc.
Non-conformances, deviations, waivers: Intake, evaluate, resolve, and track product and process nonconformance with integrated change management
Risk and reliability: FMEA with direct connection of DFMEA to BOM, Weibull life data analysis, fault tree analysis, risk-based design, prediction, and critical to quality
Standards and compliance: ISO9000, Six Sigma, APQP, CMMI, and medical device standards like FDA 21 CFR Part 820
Medical device applicability: Pre-configured ISO 13485 processes
Real-time connectivity: Remote monitoring of field products leveraging IoT
Customer stories
PTC customers have reaped the rewards that come with better quality management in industries ranging from life sciences to electronics and high tech. See for yourself.
Analyst report: Beyond ordinary PLM
Learn best practices from outstanding organizations on how to leverage a holistic, quality-first PLM strategy.
CIMdata’s closed-loop quality tool
Get specific recommendations on capabilities that will help you meet your quality management initiatives.
Windchill risk and reliability free trial
Check out our fully integrated software suite, considered the industry's most powerful reliability analysis toolkit.
The results are in, PLM-quality survey
PTC partnered with GatePoint research to find out which quality initiatives were top of mind for Manufacturing Leaders.
E-book: Driving Quality with PLM and the Digital Thread
Discover the best ways to effectively enhance quality across your enterprise with the combined power of PLM and the digital thread.