Reduce Cost of Quality and Improve Patient Safety with Digital Quality Management – a case study from CONMED
Listen to this webinar, and hear how CONMED:
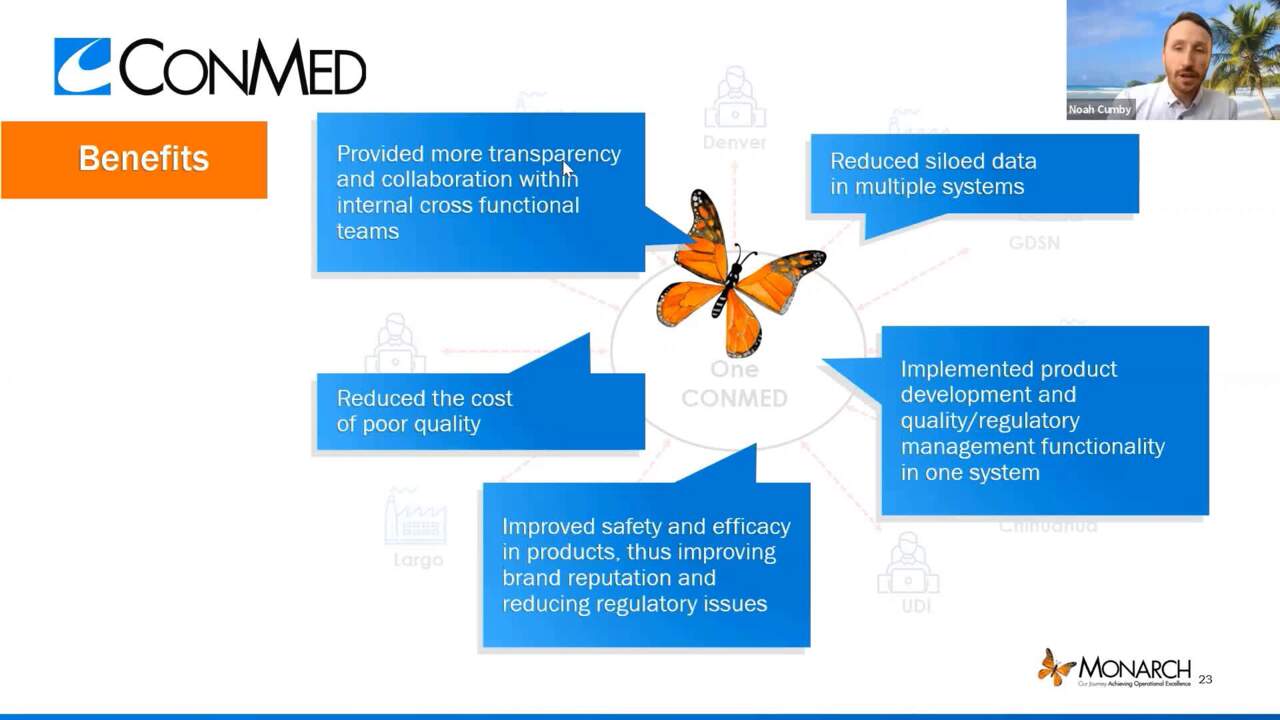
- Leveraged FDA’s CfQ to build a digital quality management transformation strategy and gain organizational alignment
- Instilled a culture of quality that is helping the organization navigate the global pandemic
- Reduced the cost of poor quality and improved operational efficiency
- Improved the safety and efficacy of products
During the session, attendees learned about enabling technologies and practical implementation approaches that will help you get started with your digital quality management initiative and work towards enterprise-scale success.
Axendia’s Market Analyst, Sandra K. Rodriguez moderated a panel discussion featuring Noah Cumby, Corporate Program Manager, QS at CONMED - David Wolf, Sr. Manager at Kalypso, – and Marc Fowler, PTC’s PLM Life Sciences Sales & Strategy Leader.
Want to learn more?
Learn More About PTC’s Quality Management solution
Build quality, reliability, safety, and process control into every part of the product lifecycle.
Build quality, reliability, safety, and process control into every part of the product lifecycle.